

#DIGESTED PROTEINS FULL#
Food remains in the stomach longer, making you feel full longer. Eating a high-protein meal increases the amount of time required to sufficiently break down the meal in the stomach. Protein digestion in the stomach takes a longer time than carbohydrate digestion, but a shorter time than fat digestion. The powerful mechanical stomach contractions churn the partially digested protein into a more uniform mixture called chyme. Egg proteins are large globular molecules and their chemical breakdown requires time and mixing. Pepsin, which is secreted by the cells that line the stomach, dismantles the protein chains into smaller and smaller fragments. The acidity of the stomach facilitates the unfolding of the proteins that still retain part of their three-dimensional structure after cooking and helps break down the protein aggregates formed during cooking.
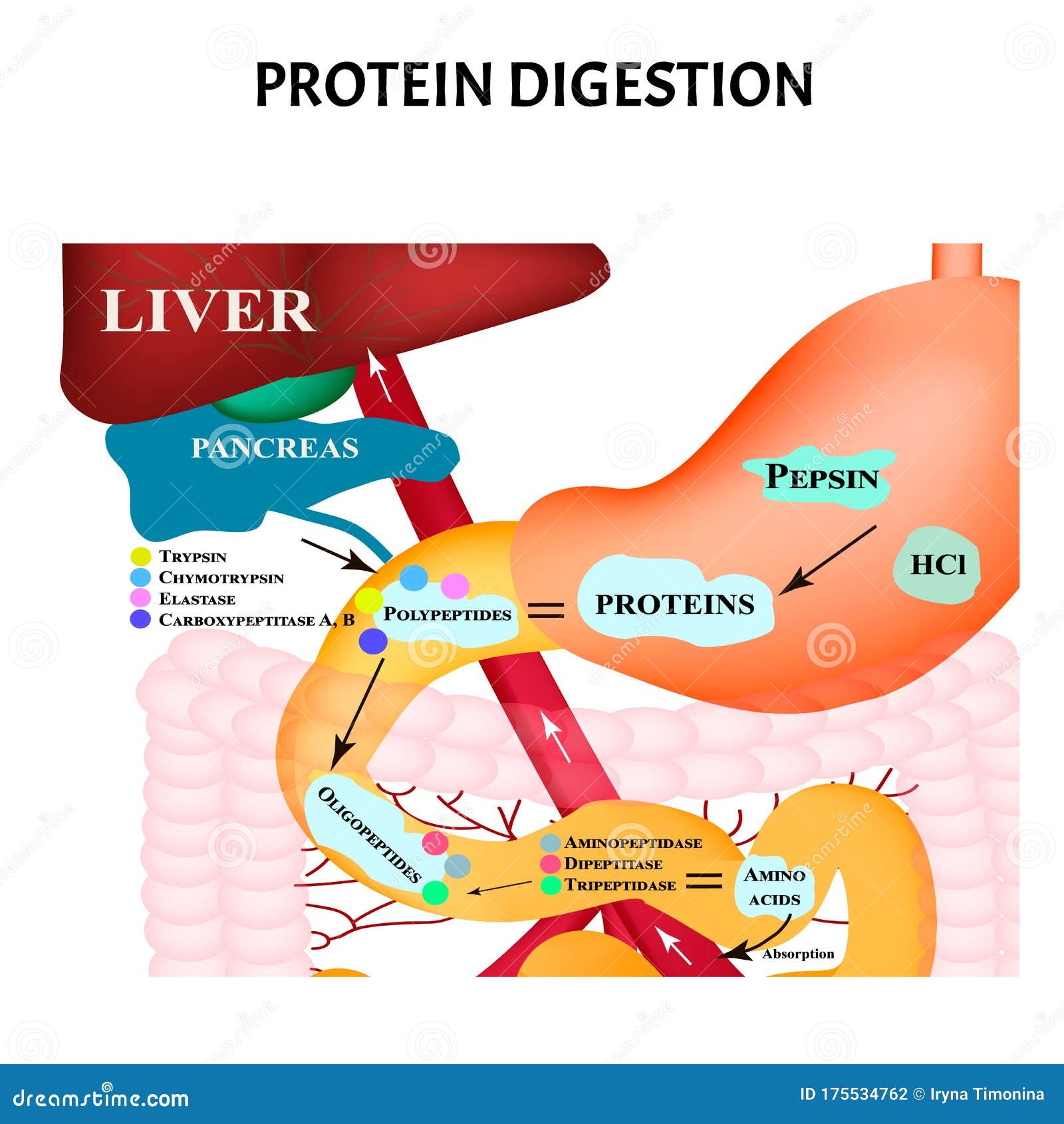
The stomach releases gastric juices containing hydrochloric acid and the enzyme, pepsin, which initiate the breakdown of the protein. The mashed egg pieces enter the stomach through the esophageal sphincter. The salivary glands provide some saliva to aid swallowing and the passage of the partially mashed egg through the esophagus. The teeth begin the mechanical breakdown of the large egg pieces into smaller pieces that can be swallowed. Unless you are eating it raw, the first step in egg digestion (or any other protein food) involves chewing. One egg, whether raw, hard-boiled, scrambled, or fried, supplies about six grams of protein.įigure 6.7 Digestion and Absorption of Protein Image by Allison Calabrese / CC BY 4.0 From the Mouth to the Stomach Eggs are a good dietary source of protein and will be used as our example to describe the path of proteins in the processes of digestion and absorption.

We previously discussed the general process of food digestion, let’s follow the specific path that proteins take down the gastrointestinal tract and into the circulatory system (Figure 6.7 “Digestion and Absorption of Protein”). How do the proteins from foods, denatured or not, get processed into amino acids that cells can use to make new proteins? When you eat food the body’s digestive system breaks down the protein into the individual amino acids, which are absorbed and used by cells to build other proteins and a few other macromolecules, such as DNA. The unraveled protein strands then stick together, forming an aggregate (or network).įigure 6.6 Protein Denaturation When a protein is exposed to a different environment, such as increased temperature, it unfolds into a single strand of amino acids. This destroys the weak bonds holding proteins in their complex shape (though this does not happen to the stronger peptide bonds). During cooking the applied heat causes proteins to vibrate. Because proteins’ function is dependent on their shape, denatured proteins are no longer functional. Weak chemical forces that hold tertiary and secondary protein structures together are broken when a protein is exposed to unnatural conditions. When a protein denatures, its complicated folded structure unravels, and it becomes just a long strand of amino acids again. Heat, acid, high salt concentrations, alcohol, and mechanical agitation can cause proteins to denature. Denaturation refers to the physical changes that take place in a protein exposed to abnormal conditions in the environment. When food is cooked, the proteins are denatured. Protein Digestion and Absorption Protein Denaturation: Unraveling the Fold


 0 kommentar(er)
0 kommentar(er)
